New chemical methods are expected to overcome the drawbacks of lithium-air batteries.
Lithium-air batteries provide more power at the same weight and are therefore widely used in electric vehicles and other mobile electronic devices. However, lithium-air batteries have a number of disadvantages: high heat generation (a large amount of chemical energy is converted into heat without being supplied to the equipment), short life, expensive and complicated manufacturing processes (expensive equipment is required to charge and discharge air into the battery, and very troublesome).
Recently, Li Ju, a professor of nuclear engineering at the Massachusetts Institute of Technology, and Zhu Zhu, a postdoctoral fellow, and five other authors working at MIT, Argonne National Laboratory, and Peking University, published articles in the journal Nature and Energy. A new technology called "nanolithium cathode" has been reported, which can be used to fabricate a closed lithium battery with similar performance to a lithium-air battery to overcome the above problems.
Li Ju said that one of the weaknesses of lithium-air batteries is that the charging and discharging voltages are not equal, and the discharging voltage is 1.2 volts lower than the charging voltage. Therefore, the energy lost in the form of heat energy is as high as 30% during each charging process. "If you charge too fast, your lithium battery is really likely to burn."
Lock up the gas
When a conventional lithium-air battery is discharged, it uses a gas in the air to chemically react with lithium in the battery. At the time of charging, oxygen in the lithium oxide is released to the atmosphere.
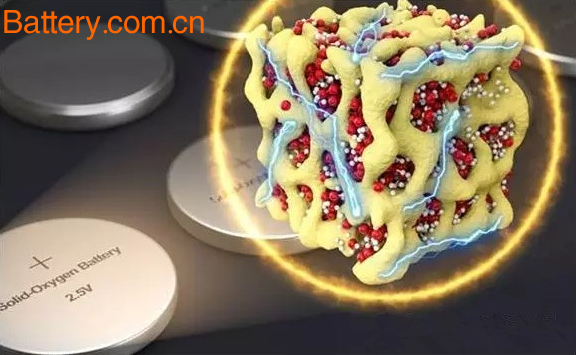
The new nanolithium cathode battery also utilizes the action of oxygen and lithium. The difference is that, when discharged, oxygen is no longer released as a gas, but is enclosed in a solid. Specifically, oxygen is enclosed in three solid compounds in three redox states - Li2O, Li2O2, and LiO2, which are in turn surrounded by cobalt dioxide glass.
The advantage of this is that the nano-lithium cathode battery reduces the voltage difference between charge and discharge by a factor of five - from 1.2 volts to 0.24 volts, so only 8% of the energy is lost as heat during charging. This means that car batteries can be charged at a faster rate without worrying about overheating.
A second disadvantage of lithium-air batteries is that the oxygen released in the form of a gas destroys the ion channels in the battery, thus reducing the life of the lithium battery.
The new technology encapsulates lithium and oxygen in a nanoscale space with cobalt dioxide glass, which scientists call nanolithium. Thus, the transformation of LiO2, Li2O2, and Li2O does not change the macrostructure of the material. In addition, since the nano-lithium particles themselves are very unstable, cobalt dioxide glasses with nanochannels are just enough to stabilize them. Cobalt dioxide also plays a catalytic role in charge and discharge.
Li Ju said that the traditional lithium-air battery is actually only a lithium-oxygen battery, because the water vapor and carbon dioxide in the air will react with lithium, so the lithium-air battery must be equipped with a huge auxiliary system to remove water vapor. And carbon dioxide, this is a very difficult step. The new solid lithium battery has no such problem.
Comes with anti-overcharge function
The new solid-state lithium battery also comes with an overcharge prevention function because the chemical reaction in the battery is self-regulated by negative feedback, and when the charge is full, the reaction stops automatically. If the traditional battery is overcharged, it will cause irreversible damage to the battery and even explode. However, the solid lithium battery was continuously charged for 15 days in the experiment, and the charging power was hundreds of times the battery capacity, but the battery was unscathed.
In the charge and discharge experiments, the solid lithium battery was charged and discharged 120 times, and the capacity was only lost by 2%, which means far exceeding the life of the conventional lithium-air battery. Because solid lithium batteries do not require an auxiliary air purification system, they can be easily applied to electric vehicles and even grid-level energy storage stations.
Since the solid oxygen cathode is much lighter than the cathode of a lithium ion battery, the new solid lithium battery can store twice as much electricity as a lithium ion battery of the same weight. As technology improves, the power of new batteries can be doubled.
The bigger advantage of the new battery is that it does not require the use of expensive and rare materials. The carbonate electrolyte of this battery is very cheap. In addition, cobalt dioxide glass is 50% lighter than nano lithium particles. Therefore, compared to lithium-air batteries, new batteries are very cheap, safe, and have the potential for large-scale applications. The research team plans to complete the manufacture of a practical prototype within one year.
Xiulei Lei, an associate professor at Oregon State University who did not participate in the project, said that the performance of the new lithium battery technology has made significant progress compared to the traditional lithium-air battery. Superoxide solutes work well in carbonate dielectrics without the need for oxygen intervention. During the charging and discharging process, the cathode material is solid, so the new battery is easy to install and use.